
The Story of Carbon
Carbon. No climate conversation is ever complete without mentioning this one element of our periodic table. Carbon emissions, carbon fuel, carbon footprint, carbon sink, carbon cycle, carbon capturing, carbon credit this single element is present in so many narratives around Climate Change, be it cause, effect or solutions. It's only imperative that I delve into the story of carbon deeply.
Carbon is everywhere. Look around you, almost anything you can lay your eyes on, has carbon in it. Wood, all types of plastic, sugar, plants, cotton, synthetic cloth, paper, fuel in our cars, the food we eat, the gas with which that food is cooked, our body, our skin, the lotion we put on that skin, everything contains carbon. Carbon is promiscuous, forms the most number of compounds than any other element, that's why its presence is abundant in our lives.
Where did this carbon come from anyway?

All the carbon we thrive on now, wasn't always the property of earth. When our planet first formed around 4.54 billion years ago, the temperatures were more than 3600° Fahrenheit. The planet was basically molten metals and minerals at such a high temperature. Most of the volatile elements like carbon, sulfur, nitrogen just boiled away into space.
At that time, the planet was also colliding with a lot of rocky debris and asteroids which were rich in carbon, sulfur, nitrogen and other elements. When the temperatures started to finally drop, the earth's core began to solidify and the rocky crust formed, these elements began to accumulate on the surface, delivered by these carbonaceous asteroid collisions.
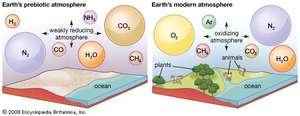
Before life sprung out of this carbon, much of this carbon was in our atmosphere as carbon dioxide. When the oceans formed, this CO₂ started getting absorbed in water. Eventually, simple bacteria came into existence in water, feeding off CO₂ and sunlight, releasing oxygen as waste. This way, the CO₂ level started dropping in the atmosphere while oxygen started increasing. This made oxygen based life possible on land. A cycle was developed, where the ocean and plants would take up CO₂, releasing oxygen and all the living creatures will breathe oxygen, releasing CO₂. There was one more way the excess carbon was cleared up from the air.
How Fossil fuel enabled us to exist...
Around 360-299 million years ago, even before the age of dinosaurs, the land was covered in swamps, filled with organisms and plants. As they died, they sank into the bottom of these swamps and got covered under layers of sand and minerals. Over millions of years, they decomposed and turned into what we call fossil fuel these days. This whole period is called the Carboniferous period, where a lot of carbon was sort of captured from the air and stored under the land.
Different types of fossil fuel formed depending on the organic matter, time, temperature and pressure condition of decomposing. Plants and trees turned into coal while small organisms like zooplankton and algae turned into crude oil and into gas if subjected to more temperature and pressure.
So now, after millions of years of processes, earth has settled into this habitable atmosphere where O₂ and N₂ make up 99% of our air, while CO₂ is just 0.04% and our temperature is nice and cozy 58° Fahrenheit. What would've happened if this process hadn't taken place? Well, we don't need to look very far, our friendly neighbor Venus can demonstrate that to us, who have 96% CO₂ in its atmosphere and 900° Fahrenheit surface temperature. Since CO₂ is a greenhouse gas, it absorbs a lot of solar radiation, making Venus a hothouse.
Too little CO₂ isn't a good thing either, if there's no CO₂ in our atmosphere, all the solar radiation would just bounce back into the space and all of earth's water will freeze up. Between these two extremes, comes our current situation where there is just right CO₂ in the air, keeping the planet's temperature stable for us to survive. And this balance is maintained by a process called carbon cycle. But before we explore carbon cycle, let’s first look into a rather fundamental question -
Why is CO₂ a greenhouse gas while O₂ or N₂ aren’t?
Have you ever wondered why exactly CO₂ is a greenhouse gas ? Why isn't oxygen or nitrogen trap heat the way CO₂ does ? The answer lies in these gases' molecular structure. Molecules that have just two atoms don't have much scope of any complex intramolecular movement. In a symmetrical molecule like O₂, two identical atoms of oxygen form a covalent bond by sharing their electrons with equal charge distribution.
Whereas in an asymmetrical molecule like CO₂, one atom's pull over the bonded electrons is stronger than the other, giving the molecule ability to vibrate in complex patterns. This tussle over electrons in a molecule is facilitated by the nearby infrared radiation which energizes the atoms to vibrate. This molecule then collides with another molecule and transfers that energy and the process goes on.
This way, if the concentration of these asymmetrical molecules increases in the air, this energy just gets thrown around between them, heating up the atmosphere, instead of reflecting back into the outer space.
CO₂ isn't the only greenhouse gas, other asymmetrical gases like water vapor, methane, nitrous oxide also absorb and retain the heat in the air. In fact, water vapor is more potent than CO₂ at retaining heat. Still we don't hear a lot about them because out of all these gases, our civilization is pumping out CO₂ at the highest rate.
Besides, water vapor has a way to get out of air regularly through rain or snow, there's no way to precipitate carbon dioxide in a similar fashion. Once it’s added to the atmosphere, it stays there for a long time: between 300 to 1,000 years.
Carbon cycle - the cycle of life and how humans are shattering it
Carbon cycle is a process where earth's carbon resource is exchanged continuously among the land, air, ocean and their living beings. There are mainly two types of players in this cycle - Carbon source and Carbon sink. A carbon source is something that releases carbon into the atmosphere and a carbon sink is something that removes carbon from the atmosphere.
Carbon sinks include the vegetation, oceans and soil. Plants and phytoplanktons use CO₂ in photosynthesis, storing carbon in themselves. As organisms die and decompose, some of the carbon is transferred to soil. Oceans too dissolve a lot of atmospheric CO₂ in the seawater like fizz in the soda.
In a natural carbon cycle, the only carbon sources would include organisms' respiration output and occasional forest fires. In such a system, sources and sinks would easily balance each other, the carbon concentration in air is maintained and everybody lives.

But almost all anthropogenic activities such as burning fossil fuels, deforestation, industrialization, agriculture, solid waste generation are huge carbon sources. During deforestation, we remove large quantities of stored carbon in trees out of the land, these same trees could have potentially captured more CO₂ from the air. We replace these dense forests with agricultural crops which store far less carbon.
We dig and burn the fossil fuel releasing vast amounts of carbon back into the atmosphere every day. The same fossil fuel which took millions of years to accumulate, is now getting back in the air in a flick. If we continue burning fossil fuel at the current rates, our known oil and gas reservoir will all be up in air as CO₂ in 50 years or so and our coal deposit could be gone in 150 years.
We are reversing the very process which made our existence possible millions of years ago.
With the increase in temperature this CO₂ is causing, our oceans have already started to give up. Increased temperature and acidity of seawater is causing phytoplanktons, a very key component in the ocean carbon sink, to deteriorate. We have already lost 40% of the phytoplankton population. Thus the overall absorbing ability of oceans is decreasing which means more CO₂ in the air, higher the temperature and more damage to the phytoplankton.
This is just one of those several positive feedback loops that are being triggered right now. It seems like we are deliberately closing all our carbon sinks while operating all of the carbon sources at full capacity.
Here’s where Carbon gets deadly
This is perhaps the most disturbing side of climate change. While current temperatures and other climate variables may not seem that dramatic, you’ll definitely be alarmed once you look at the CO₂ levels. As of May 2020, average atmospheric concentration of CO₂ rose to 417 ppm, which represents a 47% increase since the beginning of the industrial age, when the concentration was near 280 ppm.
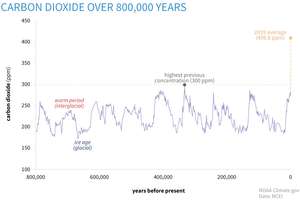
These levels are so high that even if we miraculously stopped all our emissions overnight, the temperature will still continue to rise for the foreseeable future. It can take decades to heat up the huge mass of water on our planet before the air and water temperature come in sync and surface temperature would stop rising.
In fact whatever 0.3-0.6 degrees of global warming we are looking at right now, is not the full amount of warming we can expect from the carbon dioxide we’ve already put in the environment. And let’s not forget to take into account all the positive feedback loops that are already set in the motion which will keep increasing the temperatures on their own.
That is the whole story of carbon as I’ve understood in the context of climate change. There’s a huge risk in waiting to control our emissions until global warming becomes more serious or obvious. We can’t afford to wait till we actually feel that global warming is “real” and its effects are becoming intolerable because then we really won’t be able to stop the damage. We need to get our shit together, not in the hope of recovering our past but to have a less worse future.